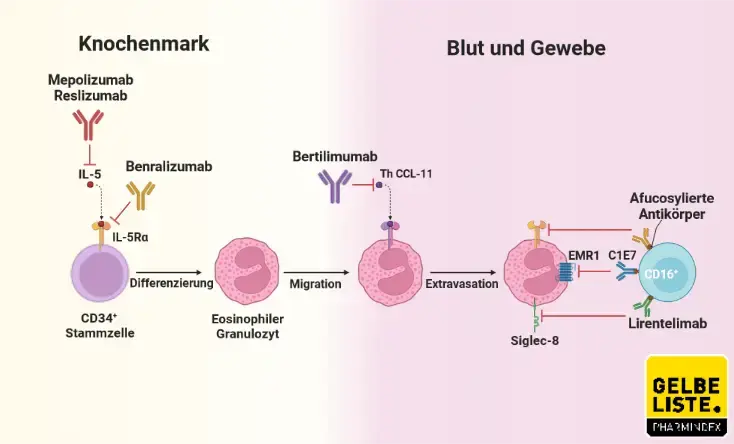
Cinqair (reslizumab) Injection for treatment of Severe Asthma and an Eosinophilic Phenotype - Clinical Trials Arena

Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials - The Lancet Respiratory Medicine
Predicting Responders to Reslizumab after 16 Weeks of Treatment Using an Algorithm Derived from Clinical Studies of Patients wit
These highlights do not include all the information needed to use CINQAIR safely and effectively. See full prescribing information for CINQAIR.CINQAIR® (reslizumab) injection, for intravenous useInitial U.S. Approval: 2016