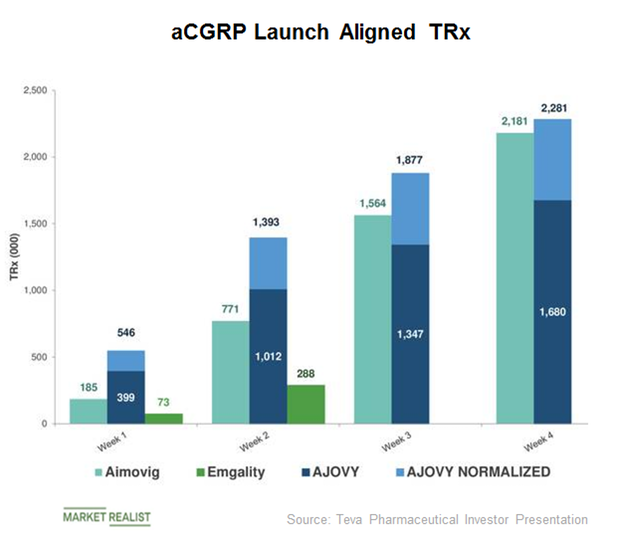
Teva's Ajovy (fremanezumab) Receives NICE Positive Recommendation for Prevention of Migraine in Adults

These highlights do not include all the information needed to use AJOVY safely and effectively. See full prescribing information for AJOVY.AJOVY® (fremanezumab-vfrm) injection, for subcutaneous useInitial U.S. Approval: 2018
Ajovy, one of four newly FDA approved migraine preventatives. Close up of product in the box Stock Photo - Alamy